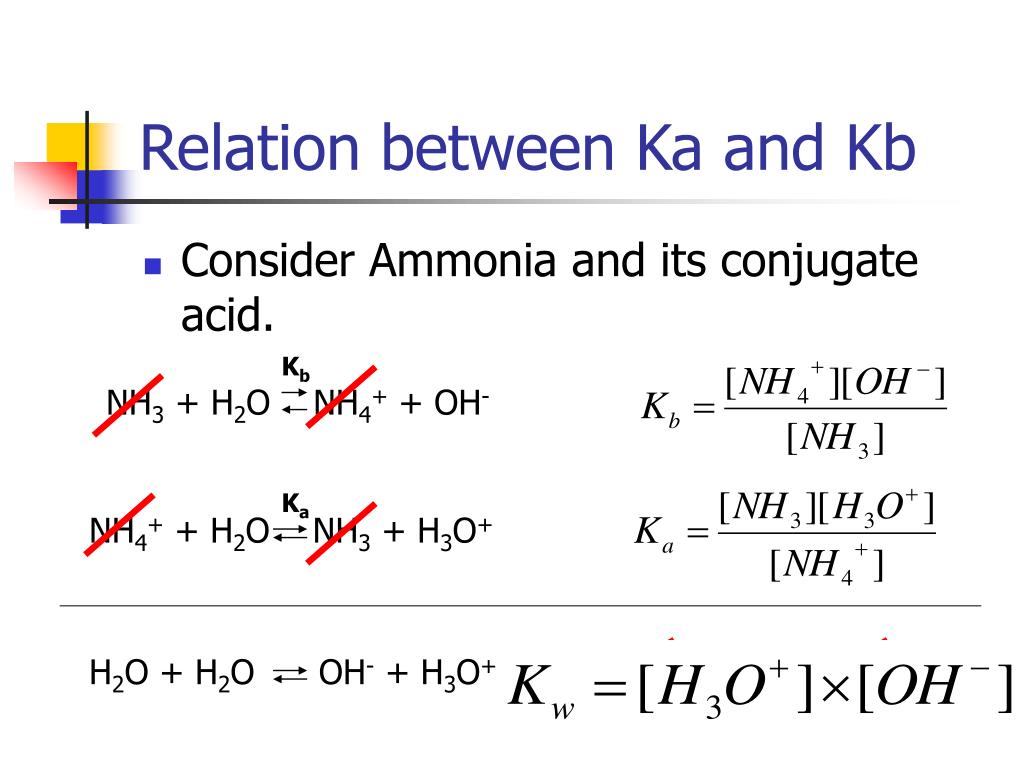
Convert Kb To Ka . First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. To find ka from kb or vice versa, you can use the ka and kb equation: An ice table is set up in order to determine the concentrations of. Divide 1x10^14 by ka.converting kb to ka. Ka = kw / kb or kb = kw / ka. Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). Ka﹒kb = kw, where kw is the autoionisation constant. ⇒ pk a + pk b = 14 at 25℃. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided.
from www.slideserve.com
An ice table is set up in order to determine the concentrations of. First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. Divide 1x10^14 by ka.converting kb to ka. ⇒ pk a + pk b = 14 at 25℃. Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). To find ka from kb or vice versa, you can use the ka and kb equation: Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. Ka﹒kb = kw, where kw is the autoionisation constant. Ka = kw / kb or kb = kw / ka. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided.
PPT Chapter 6 Problems PowerPoint Presentation, free download ID
Convert Kb To Ka First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. Divide 1x10^14 by ka.converting kb to ka. First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. Ka = kw / kb or kb = kw / ka. To find ka from kb or vice versa, you can use the ka and kb equation: Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). An ice table is set up in order to determine the concentrations of. Ka﹒kb = kw, where kw is the autoionisation constant. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. ⇒ pk a + pk b = 14 at 25℃. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and.
From softwareblade.com
Megabyte (MB) to Kilobyte (KB) Conversion Software Blade Convert Kb To Ka ⇒ pk a + pk b = 14 at 25℃. Ka = kw / kb or kb = kw / ka. First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. An ice table is set up in order to determine the concentrations of. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided.. Convert Kb To Ka.
From www.pixelconverter.com
Kb To Pixels Converter Convert Kb To Ka First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. An ice table is set up in order to determine the concentrations of. Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. Ka =. Convert Kb To Ka.
From www.youtube.com
23.2.4 Converting Between Ka and Kb YouTube Convert Kb To Ka Ka = kw / kb or kb = kw / ka. An ice table is set up in order to determine the concentrations of. ⇒ pk a + pk b = 14 at 25℃. Ka﹒kb = kw, where kw is the autoionisation constant. Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk = −log k and k =. Convert Kb To Ka.
From www.youtube.com
Ka, Kb, pKa , and pKb YouTube Convert Kb To Ka Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. ⇒ pk a + pk b = 14 at 25℃. Ka = kw / kb or kb = kw / ka. Ka﹒kb = kw, where kw is the autoionisation constant. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to. Convert Kb To Ka.
From khalidabuhakmeh.com
C Converting Bytes To Kilobytes and Beyond Khalid Abuhakmeh Convert Kb To Ka Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. Ka﹒kb = kw, where kw is the autoionisation constant. Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk =. Convert Kb To Ka.
From www.youtube.com
Converting Between Bits and Bytes "Ladder" Analogy General Maths Convert Kb To Ka First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. ⇒ pk a + pk b = 14 at 25℃. Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to. Convert Kb To Ka.
From www.vrogue.co
How To Calculate Ph Of A Solution Given Molarity vrogue.co Convert Kb To Ka Ka = kw / kb or kb = kw / ka. To find ka from kb or vice versa, you can use the ka and kb equation: Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\).. Convert Kb To Ka.
From www.courseexpert.org
(Solved) 8 Relationship Ka Kb Kal X Kb Kw 10 X 10 14 B Ka X Kbl Kw10 Convert Kb To Ka ⇒ pk a + pk b = 14 at 25℃. An ice table is set up in order to determine the concentrations of. Ka﹒kb = kw, where kw is the autoionisation constant. To find ka from kb or vice versa, you can use the ka and kb equation: Divide 1x10^14 by ka.converting kb to ka. First, the ph is used. Convert Kb To Ka.
From sciencenotes.org
pH, pKa, Ka, pKb, and Kb in Chemistry Convert Kb To Ka First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. To find ka from kb or vice versa, you can use the ka and kb equation: Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. Ka﹒kb = kw, where kw is. Convert Kb To Ka.
From proper-cooking.info
Kb Mb Gb Conversion Chart Convert Kb To Ka An ice table is set up in order to determine the concentrations of. Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). Ka﹒kb = kw, where kw is the autoionisation constant. To find ka from kb or vice versa, you can use the. Convert Kb To Ka.
From www.youtube.com
Ka and Kb Calculation YouTube Convert Kb To Ka Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. Ka = kw / kb or kb = kw. Convert Kb To Ka.
From www.ck12.org
K[a], K[b], pK[a] , and pK[b] Example 3 ( Video ) Chemistry CK12 Convert Kb To Ka Ka﹒kb = kw, where kw is the autoionisation constant. Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. Divide 1x10^14 by ka.converting kb to ka. First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. To find ka from kb or vice versa, you can use the ka and kb equation: ⇒ pk. Convert Kb To Ka.
From www.studocu.com
Ka and kb worksheet answers Studocu Convert Kb To Ka Divide 1x10^14 by ka.converting kb to ka. To find ka from kb or vice versa, you can use the ka and kb equation: An ice table is set up in order to determine the concentrations of. Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\). Convert Kb To Ka.
From www.youtube.com
Relationship between Ka and Kb for Conjugate AcidBase Pairs YouTube Convert Kb To Ka ⇒ pk a + pk b = 14 at 25℃. Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). An ice table is set up in order to determine the concentrations of. Ka = kw / kb or kb = kw / ka.. Convert Kb To Ka.
From www.youtube.com
How to Online Converter MB to KB for Image and PDF Etc... YouTube Convert Kb To Ka Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and \(pk_b\). ⇒ pk a + pk b = 14 at 25℃. Divide 1x10^14 by ka.converting kb to ka. To find ka from kb or vice versa, you can use the ka and kb equation: An. Convert Kb To Ka.
From onsheets.com
How to Convert Kb to GB in Excel 6 Best Ways On Sheets Convert Kb To Ka Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. To find ka from kb or vice versa, you can use the ka and kb equation: Use the relationships pk = −log k and k = 10 −pk (equations \(\ref{16.5.11}\) and \(\ref{16.5.13}\)) to convert between. Convert Kb To Ka.
From conniemharmonxo.blob.core.windows.net
Convert Kb To Width And Height Convert Kb To Ka Ka﹒kb = kw, where kw is the autoionisation constant. To find ka from kb or vice versa, you can use the ka and kb equation: Divide 1x10^14 by kb.yes, it's actually the same formula for both.two examples provided. ⇒ pk a + pk b = 14 at 25℃. Ka = kw / kb or kb = kw / ka. An. Convert Kb To Ka.
From www.youtube.com
How to convert between Ka and pKa (or Kb and pKb) YouTube Convert Kb To Ka Use the relationships pk = −log k and k = 10−pk (equation 16.5.11 and equation 16.5.13) to convert between \(k_a\) and \(pk_a\) or \(k_b\) and. First, the ph is used to calculate the \(\left[ \ce{h^+} \right]\) at equilibrium. Ka﹒kb = kw, where kw is the autoionisation constant. Divide 1x10^14 by ka.converting kb to ka. Use the relationships pk = −log. Convert Kb To Ka.